 Tyranny of the Weak - history book on North Korea and winner of the John K Fairbank Prize in East Asian History in 2014, criticized
Tyranny of the Weak - history book on North Korea and winner of the John K Fairbank Prize in East Asian History in 2014, criticized
Author accused of citing irrelevant or non-existing sources
references don't support material
 Recommendations to improve adverse event reporting in clinical trial publications: a joint pharmaceutical industry/journal editor perspective
Recommendations to improve adverse event reporting in clinical trial publications: a joint pharmaceutical industry/journal editor perspective
Aim to improve transparency and communication with patients
improve adverse event reporting
 Research integrity in an increasingly competitive and complex world
Research integrity in an increasingly competitive and complex world
Creating a culture of research integrity, by Irene Hames
incidence of research misconduct
 Suspicions raised over research at the University of Tokyo, Todai Research Institution
Suspicions raised over research at the University of Tokyo, Todai Research Institution
Full-fledged investigation into six professors at its medical school and the Institute of Molecular and Cellular Biosciences
biomedical research fraud
 Mega-journals: the future, a stepping stone to it or a leap into the abyss?
Mega-journals: the future, a stepping stone to it or a leap into the abyss?
Quantity, quality, peer review, rejection rates, cash cows and more....
long term future of mega-journals unclear
 Predatory conference?
Predatory conference?
Nonsense paper written by iOS autocomplete accepted for conference
nonsensical academic paper accepted
UK Research Excellence Framework blamed for dodgy research
System encourages novelty but offers no incentive for essential replication of studies to check validity of results
Research Excellence Framework discourages replication
 The US Office of Research Integrity awards research integrity grants
The US Office of Research Integrity awards research integrity grants
Projects include questionable research practices, identifying data fabrication and image manipulation
research grants available
 Iran’s scientific community shouldn’t be put in the shade
Iran’s scientific community shouldn’t be put in the shade
International editors and scholars don’t judge Iran’s large scientific community by the actions of a small “shady” minority
Iran's good research practice
 Bullied out of research
Bullied out of research
‘PhD advisers wield the power to create or destroy research careers, and students typically have few—if any—ways to protect themselves from advisers who misuse this responsibility’
misuse of power
 The rise and rise of fake news
The rise and rise of fake news
Journalists need training on how to properly verify stories and spot fakes
fake news escalates
 Case #16-06
Case #16-06
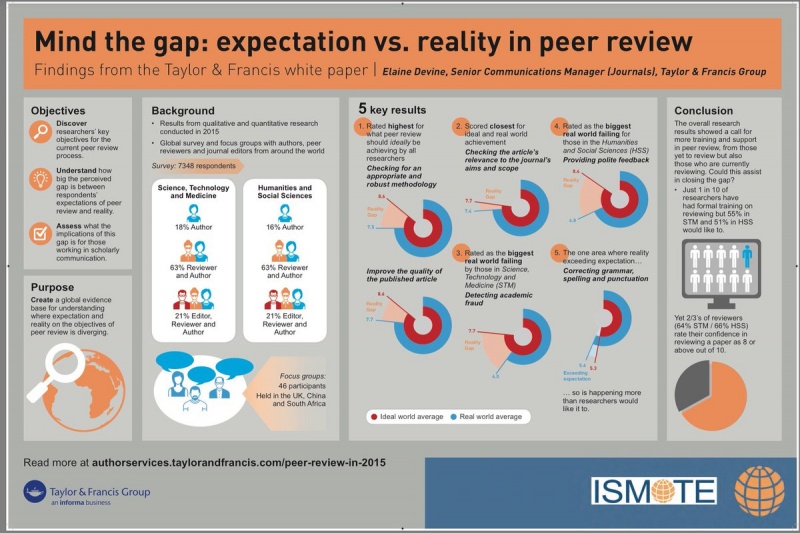

 Recommendations to improve adverse event reporting in clinical trial publications: a joint pharmaceutical industry/journal editor perspective
Recommendations to improve adverse event reporting in clinical trial publications: a joint pharmaceutical industry/journal editor perspective Research integrity in an increasingly competitive and complex world
Research integrity in an increasingly competitive and complex world Suspicions raised over research at the University of Tokyo, Todai Research Institution
Suspicions raised over research at the University of Tokyo, Todai Research Institution Mega-journals: the future, a stepping stone to it or a leap into the abyss?
Mega-journals: the future, a stepping stone to it or a leap into the abyss? Predatory conference?
Predatory conference? The US Office of Research Integrity awards research integrity grants
The US Office of Research Integrity awards research integrity grants Bullied out of research
Bullied out of research The rise and rise of fake news
The rise and rise of fake news